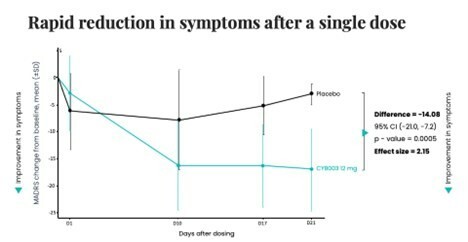
TORONTO, Oct. 31, 2023 /CNW/ - Cybin Inc. (NYSE American: CYBN) (NEO: CYBN) ("Cybin" or the "Company"), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic treatment options, today announced Phase 2 interim results for CYB003, its proprietary deuterated psilocybin analog, demonstrating a rapid, robust and statistically significant reduction in symptoms of depression three weeks following a single 12mg dose compared to placebo. At the 3-week primary efficacy endpoint, the reduction in major depressive disorder ("MDD") symptoms, definedas change from baseline in MADRS total score, was superior in participants assigned to CYB003 compared to the participants who received placebo by 14.08 points (p=0.0005, Cohen's d=2.15). A p-value indicates statistical significance. Generally, values <0.05 are considered statistically significant and values <0.001 are considered highly statistically significant.
"The overwhelmingly positive interim results for the 12mg dose of CYB003 are extremely encouraging for patients and providers. The efficacy demonstrated at that dosage showed an unprecedented reduction in depressive symptoms compared to currently available treatments," said Doug Drysdale, Chief Executive Officer of Cybin. "With these encouraging results in hand, we look forward to sharing the full complement of topline data later this quarter, and 12-week durability data in the first quarter of 2024. Our planning continues as we prepare for a larger international, multisite Phase 3 trial in early 2024 to further evaluate the safety and efficacy of CYB003 in people suffering from MDD."
The Phase 2 clinical trial is evaluating efficacy using the MADRS scale, with a primary efficacy endpoint of reduction in depression symptoms (change from baseline in MADRS) at week 3 after a single administration. To date, dosing has been completed in all dose cohorts up to 16mg, with a favorable safety and tolerability profile and no treatment-related serious adverse events observed. Interim results from the 12mg dose cohort have demonstrated a statistically significant and clinically meaningful reduction in symptoms of depression with a single dose at three weeks after treatment.
The MADRS is a 10-item, clinician-administered scale designed to measure overall severity of depressive symptoms in subjects with MDD. It is widely used in clinical trials and accepted by regulatory authorities worldwide as a measure of symptoms of depression. The MADRS includes items ranging from sadness of mood, reduction in sleep and appetite, to difficulties in concentration, anhedonia, and negative and suicidal thoughts that are scored from 0 to 6 giving a total score ranging from 0 to 60. Typical score ranges for severity are: 0-6 normal; 7-19 mild; 20-34 moderate; and >34 severe depression. In the CYB003 study, mean baseline total scores on the MADRS were 32.6 and 33.3 in the active and placebo groups, respectively.
Summary of CYB003 12mg interim efficacy data at three weeks:
Safety and tolerability:
"These positive interim safety and efficacy results support progressing to pivotal studies. We plan to request an end of Phase 2 meeting with the FDA in early 2024 to align on Phase 3 trial design, and we are commencing dosing with a capsule formulation of CYB003 in the bioequivalence cohort and further manufacturing of GMP materials that will be dose flexible, patient friendly and commercially scalable. This is an exciting time – not only for Cybin, but for the entire psychedelics sector – as we now have interim results showing a significant improvement in depressive symptoms with a single dose, moving us ever closer to delivering on our mission to improve the treatment landscape across the spectrum of mental health disorders," concluded Drysdale.
Cybin's Chief Medical Officer, Amir Inamdar said, "Mental health disorders affect almost 1 billion people worldwide. Comorbid MDD occurs widely in medical and psychiatric disorders, including anxiety disorders and post-traumatic stress disorder. These interim results, together with emerging data from a number of academic studies, suggest that CYB003 may have therapeutic efficacy in range of mental health conditions."
Full topline safety and efficacy data from the CYB003 MDD study is expected by the end Q4 2023, with 12-week durability data anticipated in Q1 2024. Cybin plans to submit this topline data to the FDA and request an end of Phase 2 meeting to be held in Q1 2024. Recruiting for a CYB003 Phase 3 study is anticipated to begin by the end of Q1 2024.
The Company also expects to share topline Phase 1 data for CYB004 and SPL028, its proprietary novel deuterated N,N-dimethyltryptamine ("DMT") compounds, before the end of 2023, supporting the initiation of a Phase 2 study in participants with generalized anxiety disorder in Q1 2024.
Conference Call and Webcast Details: | |
Date: | Wednesday, November 1, 2023 |
Time: | 11:00 a.m. ET. |
Dial-in: | 800-245-3047 (U.S. Toll-Free) or 203-518-9765 (International) |
Conference ID: | CYBN1101 |
Webcast: | Register for the webcast here |
The archived webcast will also be available on Cybin's investor relations website on the Events & Presentations page.
The Phase 1/2 trial is a randomized, double-blind, placebo-controlled study evaluating CYB003 in participants with moderate to severe MDD and in healthy volunteers. Participants with MDD received two administrations (placebo/active and active/active) three weeks apart and response/remission are assessed three weeks after each dose. MDD participants in the trial that are currently being treated with antidepressants are allowed to remain on their antidepressant medication.
The study is evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics, and psychedelic effect of ascending oral doses of CYB003. In participants with MDD, the trial evaluates rapid onset of antidepressant effect on the day of dosing, using MADRS to evaluate the incremental benefit of a second dose of CYB003 when administered at Week 3. An optional period of assessment will help determine the durability of treatment effect out to 12 weeks. The study is listed on ClinicalTrials.gov under Identifier: NCT05385783.
Cybin is a clinical-stage biopharmaceutical company on a mission to create safe and effective psychedelic-based therapeutics to address the large unmet need for new and innovative treatment options for people who suffer from mental health conditions.
Cybin's goal of revolutionizing mental healthcare is supported by a network of world-class partners and internationally recognized scientists aimed at progressing proprietary drug discovery platforms, innovative drug delivery systems, and novel formulation approaches and treatment regimens. The Company is currently developing CYB003, a proprietary deuterated psilocybin analog for the treatment of major depressive disorder and CYB004, a proprietary deuterated DMT molecule for generalized anxiety disorder and has a research pipeline of investigational psychedelic-based compounds.
Headquartered in Canada and founded in 2019, Cybin is operational in Canada, the United States, the United Kingdom, the Netherlands and Ireland. For company updates and to learn more about Cybin, visit www.cybin.com or follow the team on X, LinkedIn, YouTube and Instagram.
Certain statements in this news release relating to the Company are forward-looking statements and are prospective in nature. Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as "may", "should", "could", "intend", "estimate", "plan", "anticipate", "expect", "believe" or "continue", or the negative thereof or similar variations. Forward-looking statements in this news release include statements regarding Cybin's plans to report Phase 2 safety and efficacy data from its CYB003 deuterated psilocybin analog program in late 2023; release of CYB003 12-week durability data in Q1 2024; progression to Phase 3 development of CYB003 in early 2024; recruitment for a CYB003 phase 3 study and commencement of the phase 3 study in late Q1 2024; the Company's plan to request an end of Phase 2 meeting with the FDA in early 2024; topline Phase 1 data for CYB004 and SPL028, the Company's proprietary novel deuterated DMT compounds, before the end of 2023; commencement of a phase 2 study of the Company's proprietary novel deuterated DMT compounds in patients with generalized anxiety disorder in Q1 2024; and the Company's proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens for mental health disorders.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the spread of COVID-19 on the Company's operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in each of the Company's management's discussion and analysis for the three months ended June 30, 2023, and the Company's annual information form for the year ended March 31, 2023, which are available under the Company's profile on SEDAR+ at www.sedarplus.ca and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Although the forward-looking statements contained in this news release are based upon what management of the Company believes, or believed at the time, to be reasonable assumptions, the Company cannot assure shareholders that actual results will be consistent with such forward-looking statements, as there may be other factors that cause results not to be as anticipated, estimated or intended. Readers should not place undue reliance on the forward-looking statements and information contained in this news release. The Company assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change, except as required by law.
Cybin makes no medical, treatment or health benefit claims about Cybin's proposed products. The U.S. Food and Drug Administration, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds. The efficacy of such products has not been confirmed by approved research. There is no assurance that the use of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds can diagnose, treat, cure or prevent any disease or condition. Rigorous scientific research and clinical trials are needed. Cybin has not conducted clinical trials for the use of its proposed products. Any references to quality, consistency, efficacy and safety of potential products do not imply that Cybin verified such in clinical trials or that Cybin will complete such trials. If Cybin cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Cybin's performance and operations.
Neither the Neo Exchange Inc. nor the NYSE American LLC stock exchange have approved or disapproved the contents of this news release and are not responsible for the adequacy and accuracy of the contents herein.
Footnotes
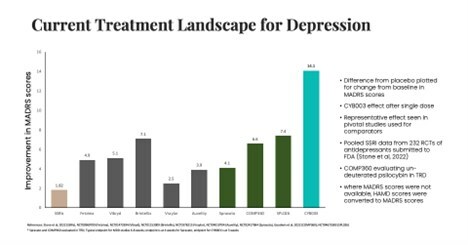
1. | FETZIMA® is a registered trademark of Allergan Sales, LLC, an AbbVie company. VIIBRYD® is a registered trademark of Allergan Sales, LLC, an AbbVie company. TRINTELLIX®, formerly BRINTELLIX, is a trademark of H. Lundbeck A/S registered with the U.S. Patent and Trademark Office and used under license by Takeda Pharmaceuticals America, Inc. VRAYLAR® is a trademark of Allergan Pharmaceuticals International Limited, an AbbVie company. AUVELITY® is a registered trademark of Axsome Therapeutics, Inc. SPRAVATO® is a registered trademark of Johnson & Johnson Corporation. Third party trademarks used herein are trademarks of their respective owners. |

| Last Trade: | US$9.61 |
| Daily Change: | 0.29 3.11 |
| Daily Volume: | 570,109 |
| Market Cap: | US$192.100M |
November 18, 2024 October 24, 2024 October 01, 2024 August 13, 2024 | |

UGE International develops, owns, and operates commercial and community solar projects in the United States and strategic markets abroad. Our distributed energy solutions deliver cheaper, cleaner energy to businesses and consumers...
CLICK TO LEARN MORE
Leveraging its vertically-integrated approach from mine to material manufacturing, Graphite One intends to produce high-grade anode material for the lithium-ion electric vehicle battery market and energy storage systems...
CLICK TO LEARN MORECOPYRIGHT ©2022 GREEN STOCK NEWS