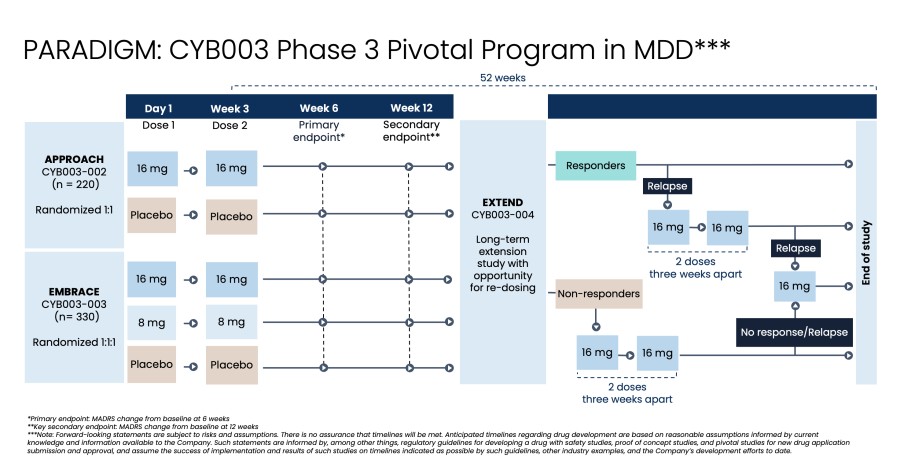
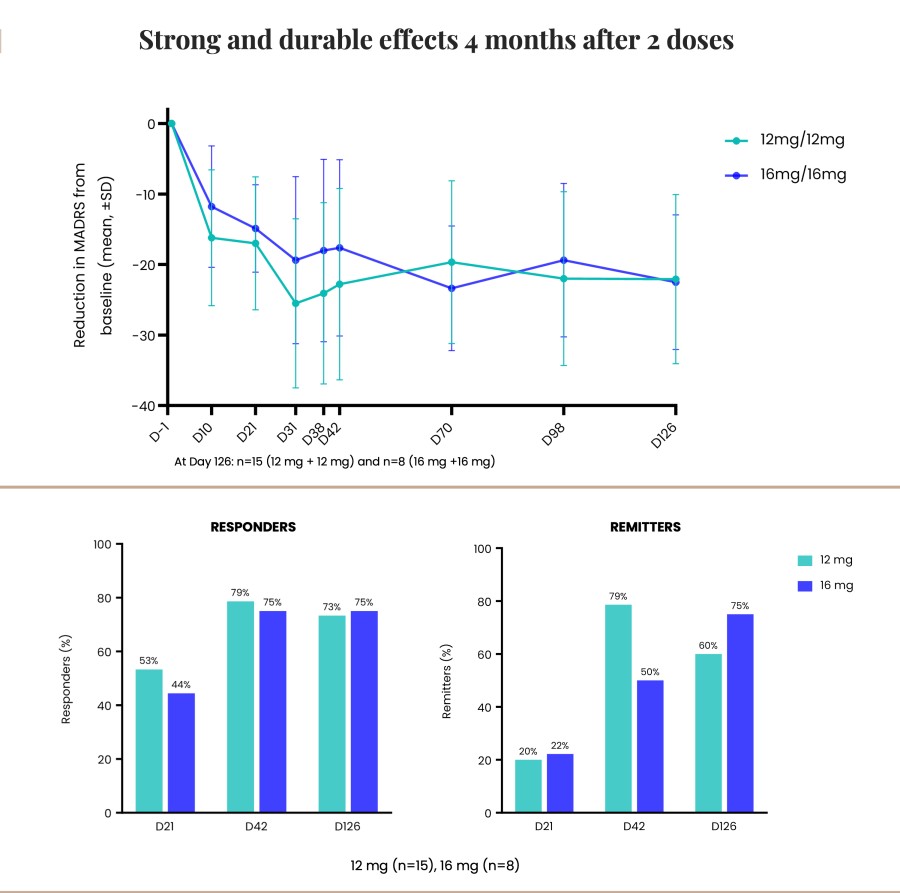
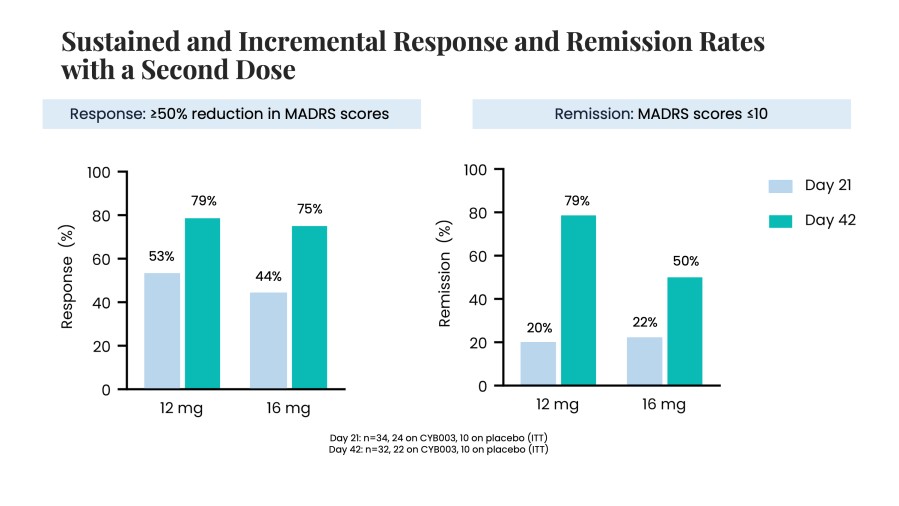
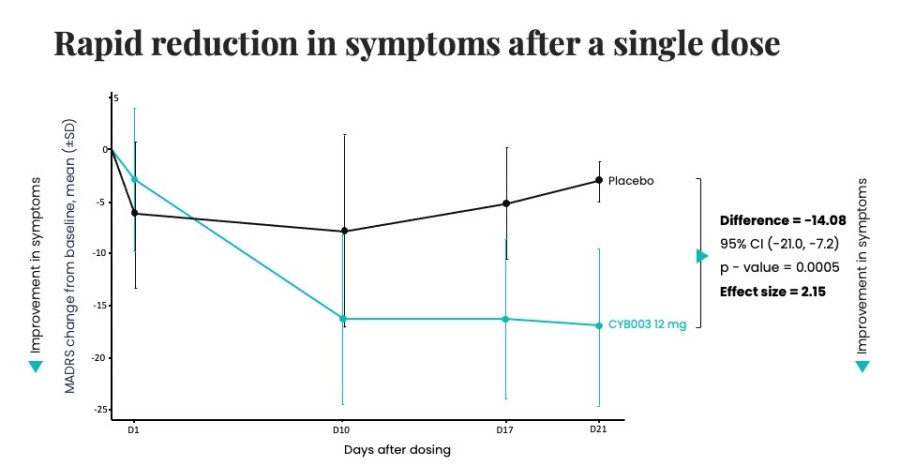
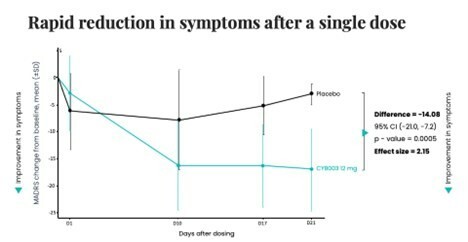
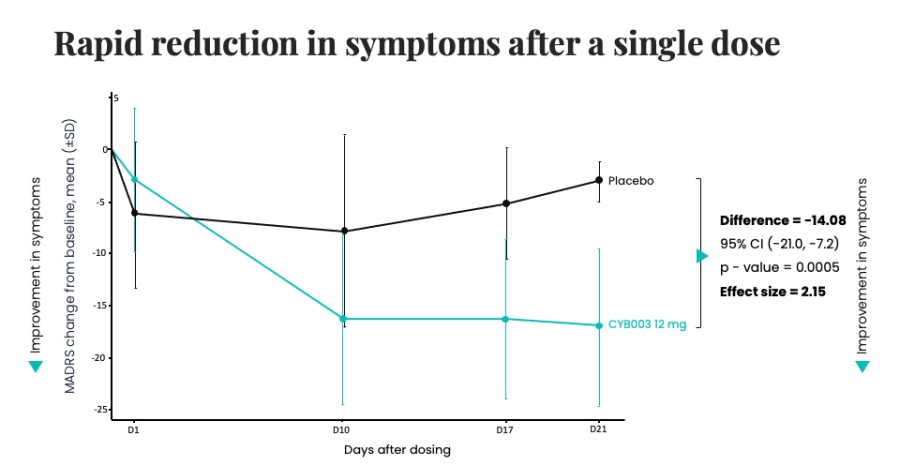
Poster presentations highlight clinical data across Cybin’s CYB003 deuterated psilocin and DMT programs TORONTO / Dec 10, 2024 / Business Wire / Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“ Cybin ” or the “ Company ”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the presentation of... Read More