Achieves Higher Gross Margin, Moves Closer to Profitability
TORONTO, Nov. 14, 2023 /PRNewswire/ - MediPharm Labs Corp. (TSX: LABS) (OTCQB: MEDIF) (FSE: MLZ) ("MediPharm", "MediPharm Labs" or the "Company") a pharmaceutical company specialized in precision-based cannabinoids, today announced its financial results for the three months ended September 30, 2023.
Third Quarter 2023 – Select Highlights
Groundwork for Growth in International Medical Cannabis
Continued Progress Solidifying Leadership in Cannabis-Based Pharmaceutical Industry
Progress Towards Profitability
Management Commentary
David Pidduck, CEO, MediPharm Labs commented, "We are proud of our focus on margins, cost reductions and profitability. Now, our strong balance sheet and improving profitability favourably positions us to make strategic investments for revenue growth. Beyond organic growth investments, there will be many M&A opportunities to consider in the coming quarters to further grow our revenue and shorten the path to profitability."
Greg Hunter, CFO, MediPharm Labs added, "In Q3, we continued to make progress by improving gross margins, reducing expenses and reducing cash burn as we drive towards profitability. Adjusted EBITDA of negative $2.4 million improved sequentially and year over year and is our best result in over 2 years. In addition, we strengthened our balance sheet with the settlement of an outstanding dispute resulting in cash collection of $7.3 million subsequent to quarter end and as of today we have approximately $19 million in cash and less than $3 million of debt."
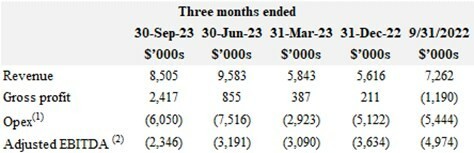
(1) Opex includes general administrative expense, marketing and selling expenses and R&D expenses. |
(2) Adjusted EBITDA is a non-IFRS measure. See "Non-IFRS Measures". |
Q3 2023 Financial Results Conference Call
MediPharm's executive management team will also host a conference call and audio webcast on Tuesday, November14, 2023 at 8:30 a.m. eastern time to discuss the Company's financial results.
Conference Call:
Toll-free number: +1 (888) 330-2454 / International number: +1 (240) 789-2714
Conference ID: 4921762
Participants are asked to dial in approximately 15 minutes before the start of the call.
Audio Webcast:
An audio webcast will be available by visiting the following link here.
For those who are unable to participate on the live conference call or webcast, a replay will be available at https://www.medipharmlabs.com/investors approximately one day after completion of the call.
About MediPharm Labs
Founded in 2015, MediPharm Labs specializes in the development and manufacture of purified, pharmaceutical-quality cannabis concentrates, active pharmaceutical ingredients (API) and advanced derivative products utilizing a Good Manufacturing Practices certified facility with ISO standard-built clean rooms. MediPharm Labs has invested in an expert, research driven team, state-of-the-art technology, downstream purification methodologies and purpose built facilities with five primary extraction lines for delivery of pure, trusted and precision-dosed cannabis products for its customers. Through its wholesale and white label platforms, MediPharm Labs formulates, develops (including through sensory testing), processes, packages and distributes cannabis extracts and advanced cannabinoid-based products to domestic and international markets.
In 2021, MediPharm Labs received a Pharmaceutical Drug Establishment Licence from Health Canada, becoming the only company in North America to hold a domestic Good Manufacturing Licence for the extraction of natural cannabinoids. The Company carries out its operations in compliance with all applicable laws in the countries in which it operates.
In 2023, MediPharm acquired VIVO Cannabis Inc. which expanded MediPharm's reach to medical patients in Canada via Canna Farms medical ecommerce platform, and in Australia and Germany through Beacon Medical PTY and Beacon Medical GMBH. This acquisition also included Harvest Medical Clinics in Canada which provides medical cannabis patients with Physician consultations for medical cannabis education and prescriptions.
Notes:
(1) | This is a non-IFRS reporting measure. See "Non-IFRS Measures" below. |
(2) | This is forward-looking information and based on a number of assumptions. See "Cautionary Note Regarding Forward-Looking Information" and "Assumptions". |
(3) | Based on both costs and revenue opportunities identified by MediPharm and VIVO management. Revenue opportunity assumed that both existing products may be sold into the existing sales channels of both VIVO and MediPharm. Costs savings estimated depends on the eliminating duplicated public company expenses and redundant corporate infrastructure. |
(4) | This target, and the related assumptions, involve known and unknown risks and uncertainties that may cause actual results to differ materially. While MediPharm and VIVO believe there is a reasonable basis for this target, such target may not be met. Actual results may vary and differ materially from the targets. See "Assumptions". |
(5) | Certain financial information included in this press release is neither audited nor reviewed. Where possible, the information has been constructed by management from available audited or audit reviewed financial statements. Where no audited or audit reviewed information has been available, additional management accounting information has been utilized to construct financial information. Readers are cautioned not to place undue reliance on such information. |
Assumptions
In developing the financial guidance set forth above, MediPharm has made the following assumptions and relied on the following factors and considerations:
Non-IFRS Measures
This press release contains references to "EBITDA", "Adjusted EBITDA" and "Adjusted Gross Profit", which are non-IFRS financial measures. Management believes that these supplementary non-IFRS financial measures provide useful additional information related to the operating results of the Company. These non-IFRS financial measures are not recognized under IFRS and, accordingly, users are cautioned that these measures should not be construed as alternatives to net income (loss) and gross profit determined in accordance with IFRS as measures of profitability or as alternatives to the Company's IFRS-based Financial Statements. The non-IFRS measures presented may not be comparable to similar measures presented by other issuers. EBITDA refers to earnings before interest, taxes, depreciation, and amortization and is used as an indicator of the Company's overall profitability. Adjusted EBITDA is a measure of the Company's overall financial performance and is used as an alternative to earnings or income in some circumstances. Adjusted EBITDA is essentially net income (loss) with interest, taxes, depreciation and amortization, non-cash adjustments and other unusual or non-recurring items added back. Adjusted EBITDA has limitations as an analytical tool as it does not include depreciation and amortization expense, interest income and expense, finance fees, gain in revaluation of derivative liabilities, taxes, government grants including rent and wage subsidies, one-off transactions, impairment losses on inventory and on fixed assets and intangibles, write down of deposits and share-based compensation. Because of these limitations, Adjusted EBITDA should not be considered as the sole measure of the Company's performance and should not be considered in isolation from, or as a substitute for, analysis of the Company's results as reported under IFRS. Adjusted EBITDA, as used within the Company's disclosure, may not be directly comparable to Adjusted EBITDA used by other reporting issuers. Adjusted Gross Profit refers to gross profit excluding the adjustments for biological assets, accelerated depreciation, write down of non-current deposits and write down of inventory. Adjusted Gross Profit is a useful measure as it represents gross profit for management purposes based on costs to manufacture, package and ship inventory sold, exclusive of any impairments due to changes in internal or external influences. Adjusted EBITDA and Adjusted Gross Profit do not have any standardized meanings and the Company's method of calculating such non-IFRS measures may not be comparable to calculations used by other companies bearing the same description. See "Use of Non-IFRS Measures" in the Company's management's discussion and analysis for the period ended March 31, 2023 for additional information.
Cautionary Note Regarding Forward-Looking Information
This news release contains "forward-looking information" and "forward-looking statements" (collectively, "forward-looking statements") within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as "expects", or "does not expect", "is expected", "anticipates" or "does not anticipate", "plans", "budget", "scheduled", "forecasts", "estimates", "believes" or "intends" or variations of such words and phrases or stating that certain actions, events or results "may" or "could", "would", "might" or "will" be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. In this news release, forward-looking statements relate to, among other things, statements regarding: the Company's progress toward profitability; potential annualized savings to be realized as a result of the Transaction and the Company's restructuring efforts; the anticipated timing and results of integration efforts of the Company following completion of the Transaction; potential cost synergies to be realized as a result of the Transaction; results of Investigational New Drug applications submitted to the FDA by US-based research partners; and revenue growth. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to: general business, economic, competitive, political and social uncertainties; the inability of MediPharm to obtain adequate financing; the delay or failure to receive regulatory approvals; and other factors discussed in MediPharm's filings, available on the SEDAR+ website at www.sedarplus.ca. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this news release. Except as required by law, MediPharm assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change.

| Last Trade: | US$0.05 |
| Daily Volume: | 0 |
| Market Cap: | US$21.460M |
December 04, 2025 November 13, 2025 August 14, 2025 August 13, 2025 July 24, 2025 | |
COPYRIGHT ©2025 GREEN STOCK NEWS