PITTSBURGH, April 24, 2023 (GLOBE NEWSWIRE) -- Krystal Biotech, Inc. (the “Company”) (NASDAQ: KRYS), a biotechnology company focused on developing and commercializing genetic medicines for patients with rare diseases, announced today that the Company presented new data on the compassionate use of topical beremagene geperpavec (B-VEC) to treat a patient with dystrophic epidermolysis bullosa (DEB) with recurrent cicatrizing conjunctivitis at the Association for Research in Vision and Ophthalmology (ARVO) 2023 Annual Meeting on April 23, 2023.
“DEB is a devastating disease with limited treatment options, and there is a substantial population of DEB patients with ocular complications for which treatment options are limited and often include surgery,” said Alfonso L. Sabater, M.D., PhD, Assistant Professor of Clinical Ophthalmology at the Bascom Palmer Eye Institute at the University of Miami Miller School of Medicine. “It is exciting to potentially advance a topical treatment for patients with ocular complications associated with DEB.”
The data presented describes the first application of B-VEC to treat ocular complications in a patient with DEB under a compassionate use program. The patient presented with cicatrizing conjunctivitis and underwent surgical symblepharon lysis with pannus removal in the right eye. B-VEC was administered to the patient’s right eye at regular intervals following surgery in addition to routine post-surgical management.
B-VEC was well tolerated and associated with full corneal healing by 3 months as well as significant visual acuity improvement from hand motion to 20/40 at 7 months, the latest time point of the on-going treatment effect evaluation.
A
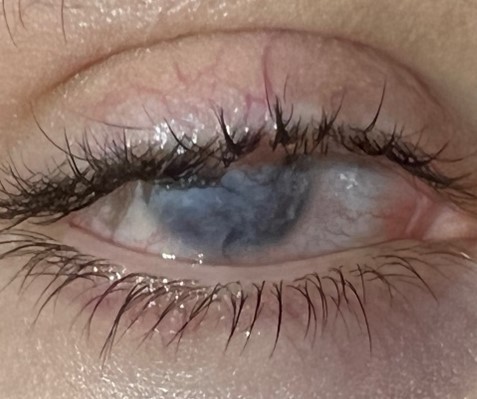
B
Figure 1: Slit lamp pictures of the right eye. A: Baseline ankyloblepharon. The visual acuity was hand motion (HM) B: Ocular surface of the right eye 6 months after the surgery after B-VEC applications.
No drug-related adverse events (AE) have been observed. Two non drug related, serious AEs were reported: 1) Prolonged hospitalization due to complications post-gastrointestinal surgery, and 2) Prolonged hospitalization due to complications post-esophageal dilation. B-VEC treatment was not interrupted during either event.
Ocular complications are common in patients with DEB, with over half of the patients diagnosed with recessive DEB potentially affected. Typical ocular manifestations include corneal abrasion, as well as corneal scarring, pannus, eyelid ectropions and blisters.1,2 There are no specific FDA-approved treatment options for ocular manifestations of DEB.3
“Ocular complications impose a heavy burden on DEB patients. Based on this promising initial data, we plan to engage with regulatory authorities and explore how we can expand the utility of B-VEC to address this urgent need,” said Suma Krishnan, President, Research & Development, Krystal Biotech. “We are also excited about the implications for our platform as this clinical data, together with ongoing preclinical studies evaluating intravitreal and subretinal routes of delivery to the eye, suggests significant potential to treat multiple ocular diseases with few or no treatment options.”
The poster was available to conference attendees and is available on the Investor section of the Company’s website.
About Dystrophic Epidermolysis Bullosa (DEB)
DEB is a rare and severe disease that affects the skin and mucosal tissues. It is caused by one or more mutations in a gene called COL7A1, which is responsible for the production of the protein type VII collagen (COL7) that forms anchoring fibrils that bind the dermis (inner layer of the skin) to the epidermis (outer layer of the skin). The lack of functional anchoring fibrils in DEB patients leads to extremely fragile skin that blisters and tears from minor friction or trauma. DEB patients suffer from open wounds, which leads to skin infections, fibrosis which can cause fusion of fingers and toes, and ultimately an increased risk of developing an aggressive form of squamous cell carcinoma which, in severe cases, can be fatal.
About B-VEC
B-VEC is an investigational non-invasive, topical, redosable gene therapy designed to deliver two copies of the COL7A1 gene when applied directly to DEB wounds. B-VEC was designed to treat DEB at the molecular level by providing the patient’s skin cells the template to make normal COL7 protein, thereby addressing the fundamental disease-causing mechanism.
The FDA and EMA have each granted B-VEC orphan drug designation for the treatment of DEB, and the FDA has granted B-VEC fast track designation and rare pediatric designation for the treatment of DEB. In addition, the FDA granted Regenerative Medicine Advanced Therapy (RMAT) to B-VEC for the treatment of DEB and the EMA granted PRIority MEdicines (PRIME) eligibility for B-VEC to treat DEB.
About Krystal Biotech, Inc.
Krystal Biotech, Inc. (NASDAQ: KRYS) is a biotechnology company focused on developing and commercializing genetic medicines for patients with rare diseases. The Company’s wide-ranging pipeline is based on its proprietary redosable HSV vector. Headquartered in Pittsburgh, Pennsylvania, the Company is led by an experienced management team, is fully-integrated and has core capabilities in viral vector design, vector optimization, gene therapy manufacturing and commercialization. For more information, please visit http://www.krystalbio.com, and follow @KrystalBiotech on LinkedIn and Twitter.
Forward-Looking Statements
Any statements in this press release about future expectations, plans and prospects for Krystal Biotech, Inc., including statements about our plans to engage with regulatory authorities to explore how we can expand the utility of B-VEC; the significant potential of our platform to treat multiple ocular diseases with few or no treatment options; and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “likely,” “will,” “would,” “could,” “should,” “continue,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of B-VEC, the sufficiency of cash resources and need for additional financing and such other important factors as are set forth under the caption “Risk Factors” in the Company’s annual and quarterly reports on file with the U.S. Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this release. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this release.
CONTACT
Investors and Media:
Meg Dodge
Krystal Biotech
This email address is being protected from spambots. You need JavaScript enabled to view it.
1. Tang JY, Marinkovich MP, Lucas E, et al. A systematic literature review of the disease burden in patients with recessive dystrophic epidermolysis bullosa. Orphanet J Rare Dis. 2021 Apr 13; 16(1): 175. doi: 10.1186/s13023-021-01811-7.
2. Tong L, Hodgkins PR, Denyer J, et al. The eye in epidermolysis bullosa. Br J Ophthalmol. 1999 Mar; 83(3): 323-6. doi:10.1136/bjo.83.3.323.
3. Chen VM, Mehta N, Robbins CC, et al. Anterior-segment spectral domain optical coherence tomography in epidermolysis bullosa. Ocul Surf. 2020 Oct; 18(4): 912-919. doi: 10.1016/j.jtos.2020.08.010

| Last Trade: | US$194.91 |
| Daily Change: | -2.51 -1.27 |
| Daily Volume: | 252,305 |
| Market Cap: | US$5.600B |
November 04, 2024 August 05, 2024 May 06, 2024 | |

Surf Air Mobility is a regional air mobility platform expanding the category of regional air travel to reinvent flying through the power of electrification. In an effort to substantially reduce the cost and environmental impact of...
CLICK TO LEARN MORE
Northstar Clean Technologies is a cleantech company focused on the sustainable recovery and reprocessing of asphalt shingles. Northstar’s mission is to be the leader in the recovery and reprocessing of asphalt shingles in North America...
CLICK TO LEARN MORECOPYRIGHT ©2022 GREEN STOCK NEWS