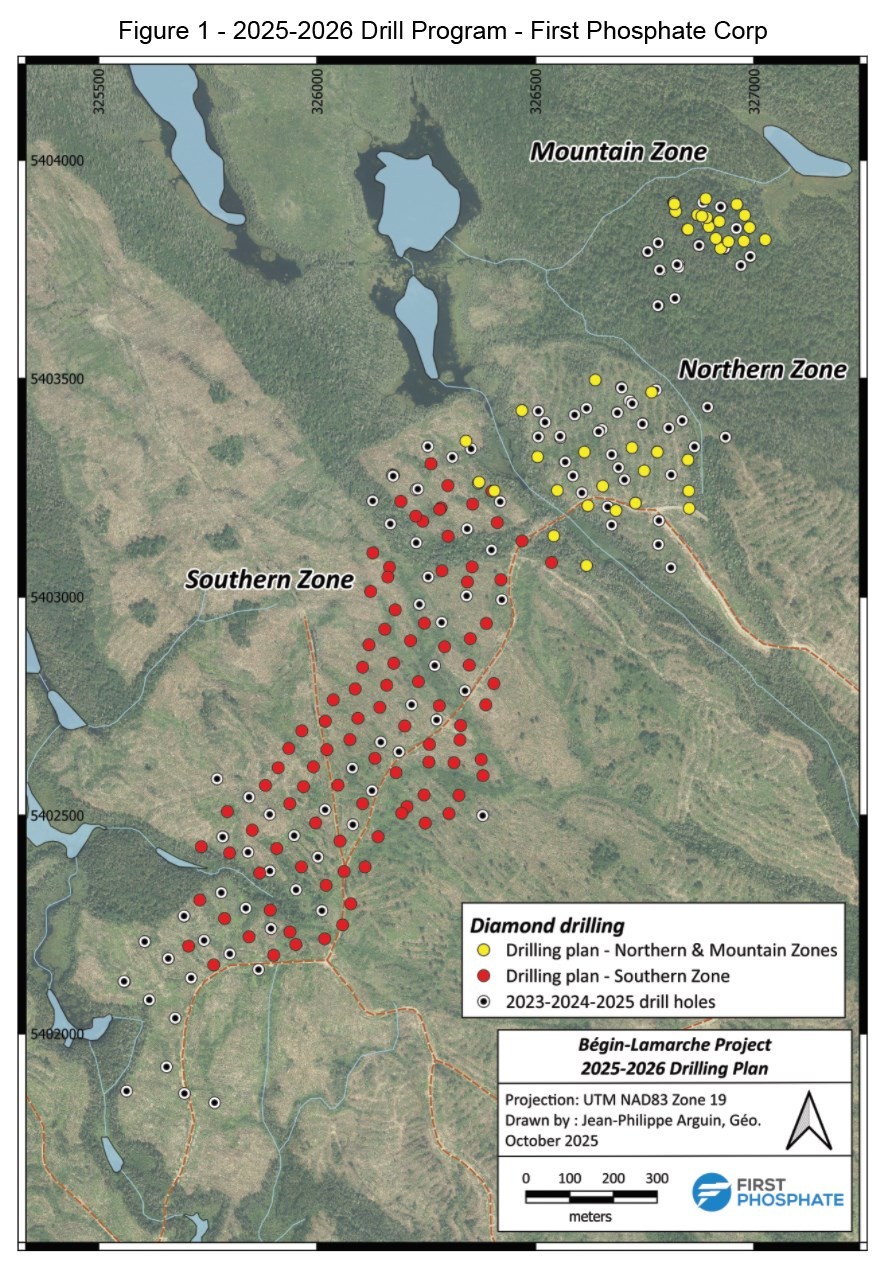
Plug Into More Green Stock News
Tap into the pulse of emerging green sectors every morning. Top daily headlines from clean energy, cleantech, cannabis, and sustainable transport stocks:
Intuitive Announces Preliminary Fourth Quarter and Full Year 2025 Results
SUNNYVALE, Calif., Jan. 14, 2026 (GLOBE NEWSWIRE) -- Intuitive (the “Company”) (Nasdaq: ISRG), a global technology leader in minimally invasive care and the pioneer of robotic-assisted surgery, today announced certain unaudited preliminary fourth quarter and full year 2025 financial results ahead of its presentation at the 44th Annual J.P. Morgan Healthcare Conference on January 14, 2026.
Financial and Operational Highlights
- Fourth quarter 2025 worldwide procedures (da Vinci and Ion combined) grew approximately 18% compared with the fourth quarter of 2024. Da Vinci procedures grew approximately 17% and Ion procedures grew approximately 44%. Average da Vinci system utilization increased 4% in the fourth quarter of 2025 as compared with the fourth quarter of 2024, primarily driven by adoption of da Vinci 5 in the U.S. and continued growth in utilization in OUS markets.
- Full year 2025 worldwide procedures (da Vinci and Ion combined) grew approximately 19% compared with 2024. Da Vinci procedures grew approximately 18% and Ion procedures grew approximately 51%. The Company expects worldwide da Vinci procedures to increase approximately 13% to 15% in 2026 as compared with 2025.
- During the fourth quarter of 2025, the Company placed 532 da Vinci surgical systems, compared with 493 in the fourth quarter of 2024. The fourth quarter 2025 da Vinci surgical system placements included 303 da Vinci 5 systems, compared with 174 in the fourth quarter of 2024. During the fourth quarter of 2025, the Company placed 42 Ion endoluminal systems, compared with 69 in the fourth quarter of 2024.
- During 2025, the Company placed 1,721 da Vinci surgical systems, compared with 1,526 systems in 2024. The 2025 da Vinci surgical system placements included 870 da Vinci 5 systems, compared with 362 in 2024. During 2025, the Company placed 195 Ion endoluminal systems, compared with 271 in 2024.
- Preliminary fourth quarter 2025 revenue of approximately $2.87 billion increased by 19% compared with $2.41 billion in the fourth quarter of 2024. Preliminary 2025 revenue of approximately $10.06 billion increased by 21% compared with $8.35 billion in 2024.
- Fourth quarter 2025 expenses included a $70 million contribution to the Intuitive Foundation compared to a $45 million contribution to the Intuitive Foundation in the fourth quarter of 2024.
Preliminary Results
The Company expects fourth quarter 2025 revenue of approximately $2.87 billion, an increase of 19% compared with $2.41 billion in the fourth quarter of 2024. The higher fourth quarter revenue was driven by growth in procedure volume, higher da Vinci system placements, and an increase in the installed base of systems. The Company expects 2025 revenue of approximately $10.06 billion, an increase of 21% compared with $8.35 billion in 2024. The unaudited results in this press release are preliminary and subject to the completion of the Company’s final closing procedures and annual independent audit and, therefore, are subject to adjustment.
Preliminary fourth quarter 2025 instruments and accessories revenue increased by 17% to approximately $1.66 billion, compared with $1.41 billion in the fourth quarter of 2024, primarily driven by approximately 18% growth in worldwide procedure volume. Preliminary full year 2025 instruments and accessories revenue increased by 19% to approximately $6.02 billion, compared with $5.08 billion for 2024, primarily driven by approximately 19% growth in worldwide procedure volume.
Fourth quarter 2025 da Vinci procedures increased approximately 17% compared with the fourth quarter of 2024. Overall, in 2025, approximately 3,153,000 surgical procedures were performed with da Vinci surgical systems, an increase of approximately 18% compared with approximately 2,683,000 surgical procedures performed with da Vinci surgical systems in 2024. The growth in the Company’s overall da Vinci procedure volume in 2025 was largely attributable to 18% growth in United States (“U.S.”) general surgery procedures as well as 23% growth in total da Vinci procedures performed outside of the U.S. (“OUS”) across a broad set of procedure specialties. The Company expects worldwide da Vinci procedures to increase approximately 13% to 15% in 2026.
Preliminary fourth quarter 2025 systems revenue was approximately $786 million, compared with $655 million in the fourth quarter of 2024. Higher systems revenue reflected increased da Vinci system placements, a higher lease installed base, and higher da Vinci system average selling prices compared with the fourth quarter of 2024. Preliminary full year 2025 systems revenue was approximately $2.47 billion, compared with $1.97 billion for 2024.
The Company placed 532 da Vinci surgical systems, of which 303 were da Vinci 5 systems, in the fourth quarter of 2025, compared with 493 systems, of which 174 were da Vinci 5 systems, in the fourth quarter of 2024. The fourth quarter 2025 da Vinci surgical system placements included 250 systems placed under operating lease arrangements, of which 150 systems were placed under usage-based operating lease arrangements, compared with 222 systems placed under operating lease arrangements, of which 140 systems were placed under usage-based operating lease arrangements in the fourth quarter of 2024.
The Company placed 1,721 da Vinci surgical systems, of which 870 were da Vinci 5 systems, in 2025, compared with 1,526 systems, of which 362 were da Vinci 5 systems, in 2024. The 2025 da Vinci surgical system placements included 872 systems placed under operating lease arrangements, of which 496 systems were placed under usage-based operating lease arrangements, compared with 776 systems placed under operating lease arrangements, of which 467 systems were placed under usage-based operating lease arrangements in 2024.
Commenting on the announcement, Intuitive CEO Dave Rosa said, “We are pleased with our strong performance in the final quarter of 2025 and the full year. In 2025, we saw increased adoption and utilization of our surgical platforms, expanded product clearances and indications for use across geographies, and more than 3.1 million da Vinci procedures performed. As always, we remain focused on delivering the goals we share with our customers, centered on improving patient care and outcomes.”
Additional unaudited preliminary revenue and procedure information has been posted to the Investor Relations section of the Intuitive website at: https://isrg.gcs-web.com/.
The Company is scheduled to present at the J.P. Morgan Healthcare Conference on January 14, 2026, at 9:00 a.m. PST. The Company is scheduled to report its fourth quarter 2025 results during a conference call on January 22, 2026, at which point the Company will discuss the 2025 financial results in more detail. Dial-in and webcast access information for both of these events are also available in the Investor Relations section of the Intuitive website.
About Intuitive
Intuitive (Nasdaq: ISRG), headquartered in Sunnyvale, California, is a global leader in minimally invasive care and the pioneer of robotic-assisted surgery. Our technologies include the da Vinci surgical systems and the Ion endoluminal system. By uniting advanced systems, progressive learning, and value-enhancing services, we help physicians and their teams optimize care delivery to support the best outcomes possible. At Intuitive, we envision a future of care that is less invasive and profoundly better, where diseases are identified early and treated quickly, so patients can get back to what matters most.
Product and brand names/logos are trademarks or registered trademarks of Intuitive or their respective owners. See www.intuitive.com/trademarks.
For more information, please visit the Company’s website at www.intuitive.com.
Forward-Looking Statements
The Company has not filed its Annual Report on Form 10-K for the year ended December 31, 2025. Accordingly, all financial results described in this press release should be considered unaudited preliminary results and are subject to change to reflect any corrections or adjustments, or changes in accounting estimates, that are identified prior to the time that the Company is in a position to complete these filings. Actual results could differ materially from these preliminary results.
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements relate to expectations concerning matters that are not historical facts. Statements using words such as “estimates,” “projects,” “believes,” “anticipates,” “plans,” “expects,” “intends,” “may,” “will,” “could,” “should,” “would,” “goals,” “seeks,” “potential,” “targeted,” and similar words and expressions are intended to identify forward-looking statements. These forward-looking statements are necessarily estimates reflecting the judgment of the Company’s management and involve a number of risks and uncertainties that could cause actual results to differ materially from those suggested by the forward-looking statements. These forward-looking statements include, but are not limited to, statements related to the Company’s unaudited preliminary financial and operational results for the fourth quarter and full year 2025, expected procedure growth in 2026, and the Company’s expectations regarding its strategic priorities, future market opportunities, and continued focus on customer goals and patient outcomes. These forward-looking statements should be considered in light of various important factors, including, but not limited to, the following: completion of the Company’s final closing procedures, final adjustments, and other developments that may arise in the course of audit and review procedures, the overall macroeconomic environment, which may impact customer spending and the Company’s costs, including tariffs, the levels of inflation, and interest rates; the conflict between Ukraine and Russia; conflicts in the Middle East; disruption to the Company’s supply chain, including difficulties in obtaining a sufficient supply of materials; curtailed or delayed capital spending by hospitals; the impact of global and regional economic and credit market conditions on healthcare spending; delays in obtaining new product approvals, clearances, or certifications from the U.S. Food and Drug Administration (“FDA”), comparable regulatory authorities, or notified bodies; the risk of the Company’s inability to comply with complex FDA and other regulations, which may result in significant enforcement actions; regulatory approvals, clearances, certifications, and restrictions or any dispute that may occur with any regulatory body; healthcare reform legislation in the U.S. and its impact on hospital spending, reimbursement, and fees levied on certain medical device revenues; changes in hospital admissions and actions by payers to limit or manage surgical procedures; the timing and success of product development and customer acceptance of developed products; the results of any collaborations, in-licensing arrangements, joint ventures, strategic alliances, or partnerships, including the joint venture with Shanghai Fosun Pharmaceutical (Group) Co., Ltd.; the Company’s completion of and ability to successfully integrate acquisitions; intellectual property positions and litigation; risks associated with the Company’s operations and any expansion outside of the U.S.; unanticipated manufacturing disruptions or the inability to meet demand for products; the Company’s reliance on sole and single sourced suppliers; the results of legal proceedings to which the Company is or may become a party; adverse publicity regarding the Company and the safety of the Company’s products and adequacy of training; the impact of changes to tax legislation, guidance, and interpretations; changes in tariffs, trade barriers, and regulatory requirements (including changes to tariffs imposed by the U.S. on imports from various countries, including Mexico, where the Company currently manufactures a significant majority of its instruments and accessories, Germany, where the Company currently manufactures a majority of its endoscopes, and China, where the Company currently imports certain materials); and other risks and uncertainties. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release and which are based on current expectations and are subject to risks, uncertainties, and assumptions that are difficult to predict, including those risk factors identified under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, as updated by the Company’s other filings with the Securities and Exchange Commission. The Company’s actual results may differ materially and adversely from those expressed in any forward-looking statement, and the Company undertakes no obligation to publicly update or release any revisions to these forward-looking statements, except as required by law.
Contact: Investor Relations
(408) 523-2161

Plug Into More Green Stock News
Tap into the pulse of emerging green sectors every morning. Top daily headlines from clean energy, cleantech, cannabis, and sustainable transport stocks:
More Green Stock News
More Green Stock News
| Last Trade: | US$536.63 |
| Daily Change: | -25.19 -4.48 |
| Daily Volume: | 1,161,424 |
| Market Cap: | US$190.240B |
December 10, 2025 October 21, 2025 September 12, 2025 September 09, 2025 | |