NEW YORK, March 30, 2023 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease, Parkinson’s disease, Rett syndrome and other Central Nervous System (CNS) disorders, reports preliminary 48-week open-label extension Parkinson’s disease dementia ANAVEX®2-73-PDD-EP-001 Phase 2 study data which demonstrated longitudinal beneficial effects of ANAVEX®2-73 on the prespecified primary and secondary objectives, as well as planned primary and key secondary endpoints which will be utilized in a forthcoming pivotal study of ANAVEX®2-73 in Parkinson’s disease.
ANAVEX®2-73 (blarcamesine) is an oral small-molecule activator of the sigma-1 receptor (SIGMAR1), which data suggests is pivotal to restoring neural cell homeostasis and promoting neuroplasticity.1
Parkinson’s disease (PD) is a chronic, debilitating CNS disease and the second largest age-related disorder after Alzheimer’s disease.2 This study demonstrates for the first-time that patients’ clinical symptoms consistently improve longitudinally during the 48-week ANAVEX2-73-PDD-EP-001 Phase 2 study under active ANAVEX®2-73 treatment in Parkinson’s disease.
The 48-week ANAVEX2-73-PDD-EP-001 (NCT04575259) Phase 2 study assessed safety, tolerability and efficacy, measuring among others, Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS)3 Parts I, II, III, REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Clinical Global Impression – Improvement (CGI-I), as well as cognitive efficacy endpoint Montreal Cognitive Assessment (MoCA) over a 48-week period.
Preliminary analysis reveals that ANAVEX®2-73 (blarcamesine) was found to be generally safe and well tolerated; and safety findings in this study are consistent with the known safety profile of ANAVEX®2-73. In respect to efficacy, across all efficacy endpoints, patients performed better while on ANAVEX®2-73.
The 48-week Open Label Extension (OLE) ANAVEX2-73-PDD-EP-001 Phase 2 study was offered to participants after completion of the double-blind placebo-controlled ANAVEX2-73-PDD-001 Phase 2 study. Study participants were allowed to stay on a stable regimen of anti-Parkinson's disease medications (including levodopa, dopamine agonists, MAO-B inhibitors, or entacapone).
Previously, in the double-blind ANAVEX2-73-PDD-001 Phase 2 study, ANAVEX®2-73 treatment demonstrated statistically significant improvements compared to placebo (ITT population) for MDS-UPDRS Total score. From baseline to the end of the trial at 14 weeks, the MDS-UPDRS Total score improved by -10.98 points in the ANAVEX®2-73 high dose group and worsened by 3.53 points in the placebo group, an adjusted mean difference of -14.51 points (p = 0.034). This corresponds to a relative improvement of 18.9% over 14 weeks.4 This data was also consistent with expression levels of pathological dysregulated neurodegenerative genes, including Parkinson’s disease genes, which were significantly restored by the therapeutic effect of ANAVEX®2-73 (p<0.005).5
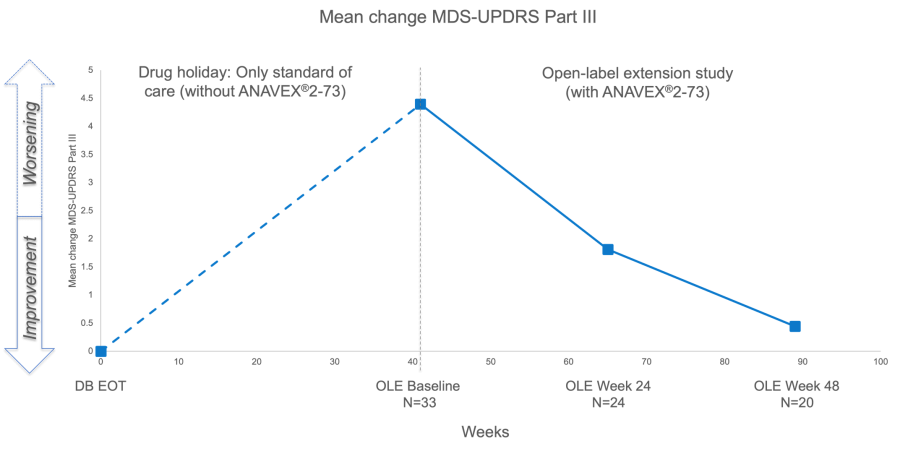
Due to the COVID-19 pandemic, the start of the extension phase was delayed, on average, by approximately 41 weeks at the end of the preceding double-blind placebo-controlled study (DB). This led to a reduced enrollment rate for the extension phase. The period between the end of the double-blind phase to the start of the extension phase, where patients were not on ANAVEX®2-73 treatment, is known as a ‘drug holiday’. The drug holiday period of treatment separation provided an opportunity to compare the trajectory of clinical scores between no ANAVEX®2-73 treatment (drug holiday) and ANAVEX®2-73 treatment in the extension phase.
All efficacy endpoints, which includes the MDS-UPDRS Part II + III and Clinical Global Impression – Improvement (CGI-I) measured at the end of trial of the double-blind study (DB EOT), the OLE Baseline, OLE Week 24, and OLE Week 48, showed a worsening during the drug holiday. However, a consistent improvement was observed during the extension phase when patients resumed ANAVEX®2-73 treatment. These results are consistent with the pattern observed for all efficacy measures in the extension phase (see Chart and Table).6
| Clinical Endpoints | Change at OLE Baseline from DB EOT | Change at Week 24 from OLE Baseline | Change at Week 48 from OLE Baseline | ||||||||
| MDS-UPDRS Part III | Mean (SE) | 4.394 (2.155) | N=33 | -2.583 (2.474) | N=24 | -3.95 (4.067) | N=20 | ||||
| Median | 0 | -0.5 | -1 | ||||||||
| MDS-UPDRS Part II + III | Mean (SE) | 6.667 (2.800) | N=33 | -3.870 (3.403) | N=23 | -2.20 (5.314) | N=20 | ||||
| Median | 0 | -1 | -0.5 | ||||||||
| MDS-UPDRS Total score | Mean (SE) | 9.818 (3.387) | N=33 | -4.739 (4.262) | N=23 | -2.25 (6.656) | N=20 | ||||
| Median | 4 | 0 | -3.5 | ||||||||
| RBDSQ | Mean (SE) | 0.784 (0.439) | N=37 | -1.667 (0.424) | N=24 | -0.524 (0.620) | N=21 | ||||
| Median | 0 | -1 | -1 | ||||||||
| CGI-I | Mean (SE) | 0.629 (0.184) | N=35 | -0.542 (0.295) | N=24 | -0.7 (0.309) | N=20 | ||||
| Median | 0 | 0 | -1 | ||||||||
| MoCA | Mean (SE) | -1.45455 (0.433) | N=33 | -0.2912 (0.519) | N=24 | -1.2 (0.537) | N=20 | ||||
| Median | -1 | 0 | -0.5 | ||||||||
| OLE = Open Label Extension study; DB = Double-Blind study; EOT = End of Trial | |||||||||||
| The calculations were done with all available data at reference time points | |||||||||||
| These results should be interpreted cautiously due to the nature of the study and the small sample sizes | |||||||||||
| MDS-UPDRS = Movement Disorder Society-Unified Parkinson Disease Rating Scale | |||||||||||
| RBDSQ = REM Sleep Behavior Disorder Screening Questionnaire | |||||||||||
| CGI-I = Clinical Global Impression – Improvement | |||||||||||
| MoCA = Montreal Cognitive Assessment (cognitive decline slowing in OLE) | |||||||||||
| Except for MoCA, Positive scores respresent decline; Negative scores represent improvement | |||||||||||
The two endpoints, MDS-UPDRS Part II + III and Clinical Global Impression – Improvement (CGI-I) measured in this study are the planned primary and key secondary endpoints in Anavex’s forthcoming pivotal 6-month Parkinson’s disease study.
“It is encouraging that the patients’ clinical symptoms consistently improved longitudinally over time during the extension phase under active ANAVEX®2-73 treatment,” said Christopher U Missling, PhD, President & CEO of Anavex. “This data suggests ANAVEX®2-73’s potential capability to slow and potentially reverse the life altering symptoms of Parkinson’s disease, an urgent unmet global need.”
Moreover, at the request of the participants completing the 48-week open-label extension study, patient requested treatment with ANAVEX®2-73 is continuing beyond the open-label 48-weeks through the compassionate use Special Access Scheme. Currently, participants in the compassionate use program for ANAVEX®2-73 have been on average, for over 2 years and counting.
The Michael J. Fox Foundation (MJFF) awarded Anavex a research grant for an imaging-focused Parkinson’s disease clinical trial with ANAVEX®2-73.7 MJFF previously awarded Anavex a research grant, which fully funded a preclinical study that established ANAVEX®2-73 as a potentially disease-modifying treatment for Parkinson’s disease.8
Anavex Life Sciences’ therapeutic product platform includes orally available small molecule lead drug candidate ANAVEX®2-73 for the treatment of Alzheimer’s disease, Parkinson’s disease and Rett syndrome and ANAVEX®3-71 for schizophrenia, Alzheimer’s disease, and frontotemporal dementia.
About Parkinson’s Disease (PD)
Parkinson’s disease is a chronic and progressive neurological disorder that is characterized by well-known motor symptoms including tremors, stiffness of limbs, slowness of movements, and difficulties with posture and balance, as well as by non-motor symptoms. It is the second most common neurological disorder and approximately one million people in the United States, and more that 10 million people worldwide, live with this disease. Parkinson’s disease is more common in people over 60 years of age and its prevalence is expected to increase significantly as the average age of the population increases. Current Parkinson’s treatments are only effective in managing symptoms of the disease, mainly through the use of levodopa and dopamine agonists. As the disease progresses and dopaminergic neurons continue to be lost, these drugs eventually become less effective at treating the symptoms.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders, including Alzheimer's disease, Parkinson's disease, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer. Anavex's lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a and recently a Phase 2b/3 clinical trial for Alzheimer's disease, a Phase 2 proof-of-concept study in Parkinson's disease dementia, and both a Phase 2 and a Phase 3 study in adult patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate that restores cellular homeostasis by targeting sigma-1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer's disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson's Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 for the treatment of Parkinson's disease. ANAVEX®3-71, which targets sigma-1 and M1 muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer's disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid, and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the company on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Investors:
Andrew J. Barwicki
Investor Relations
Tel: 516-662-9461
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
1 Advances in Experimental Medicine and Biology Volume 964 (2017) Sigma Receptors: Their Role in Disease and as Therapeutic Targets.
2 Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014 Mar;14(100):19-30. doi: 10.1016/j.arr.2014.01.004. Epub 2014 Feb 3. PMID: 24503004; PMCID: PMC3989046; Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Parkinson's disease. Subcell Biochem. 2012;65:389-455. doi: 10.1007/978-94-007-5416-4_16. PMID: 23225012; PMCID: PMC4372387.
3 The Movement Disorder Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) is a commonly used tool to measure Parkinson disease (PD) progression.
4 https://www.anavex.com/post/anavex-life-sciences-announces-presentation-of-phase-2-clinical-biomarker-data-from-pdd-study
5 https://www.anavex.com/post/anavex-announces-first-entire-clinical-alzheimer-s-gene-pathway-data-of-anavex-2-73-at-aaic-2022
6 The observed worsening (increase) of MDS-UPDRS scores in this study during drug holiday is consistent with the literature, e.g.: Holden SK, Finseth T, Sillau SH, Berman BD. Progression of MDS-UPDRS Scores Over Five Years in De Novo Parkinson Disease from the Parkinson's Progression Markers Initiative Cohort. Mov Disord Clin Pract. 2018 Jan-Feb;5(1):47-53. doi: 10.1002/mdc3.12553. Epub 2017 Sep 22; Simuni T, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, Jennings D, Tanner CM, Trojanowski JQ, Shaw LM, Seibyl J, Schuff N, Singleton A, Kieburtz K, Toga AW, Mollenhauer B, Galasko D, Chahine LM, Weintraub D, Foroud T, Tosun D, Poston K, Arnedo V, Frasier M, Sherer T, Chowdhury S, Marek K; Parkinson's Progression Marker Initiative*. Longitudinal Change of Clinical and Biological Measures in Early Parkinson's Disease: Parkinson's Progression Markers Initiative Cohort. Mov Disord. 2018 May;33(5):771-782. doi: 10.1002/mds.27361.
7 https://www.anavex.com/anavex-life-sciences-receives-michael-j-fox-foundation-grant-for-clinical-study-of-anavex2-73-blarcamesine-in-people-with-parkinsons-disease/
8 https://www.anavex.com/parkinsons-disease-data-mjf-conference/; https://www.anavex.com/anavex-awarded-grant-from-the-michael-j-fox-foundation-for-parkinsons-research/

| Last Trade: | US$10.98 |
| Daily Change: | -1.13 -9.33 |
| Daily Volume: | 2,275,746 |
| Market Cap: | US$931.100M |
December 23, 2024 December 23, 2024 December 09, 2024 November 26, 2024 November 25, 2024 | |

Leveraging its vertically-integrated approach from mine to material manufacturing, Graphite One intends to produce high-grade anode material for the lithium-ion electric vehicle battery market and energy storage systems...
CLICK TO LEARN MORE
GreenPower Motor designs, builds and distributes a full suite of high-floor and low-floor all-electric medium and heavy-duty vehicles, including transit buses, school buses, shuttles, cargo van, and a cab and chassis...
CLICK TO LEARN MORECOPYRIGHT ©2022 GREEN STOCK NEWS