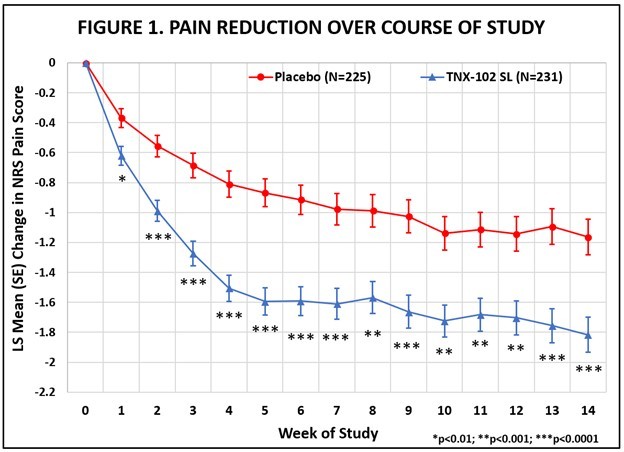
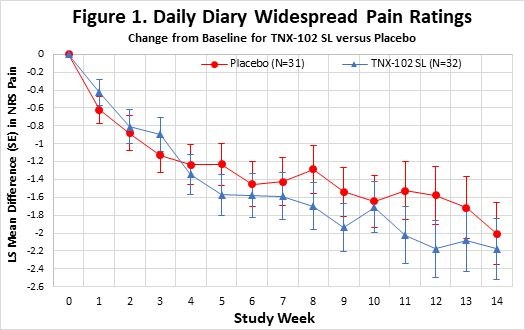
CHATHAM, N.J., Nov. 26, 2024 (GLOBE NEWSWIRE) -- Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a fully-integrated biopharmaceutical company with marketed products and a pipeline of development candidates, announced today that Jessica Morris, Chief Operating Officer of Tonix Pharmaceuticals, will present and conduct investor meetings at NobleCon20, Noble Capital Markets’ Twentieth Annual Emerging... Read More