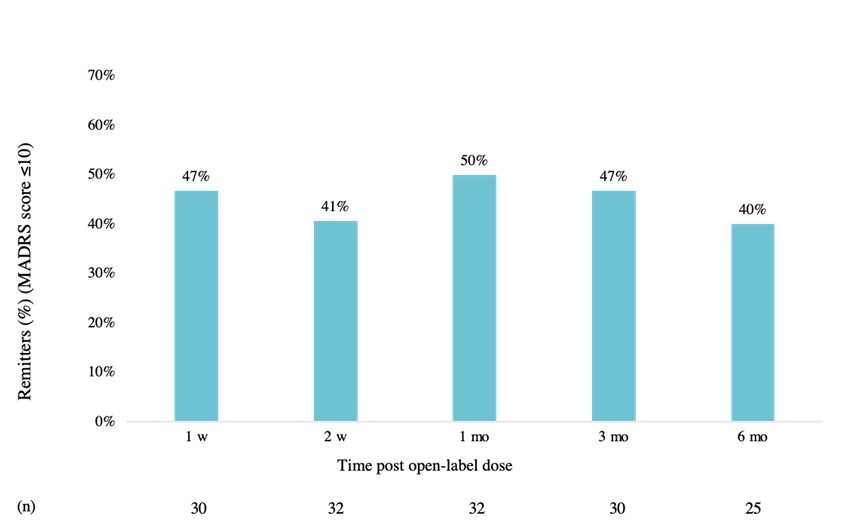
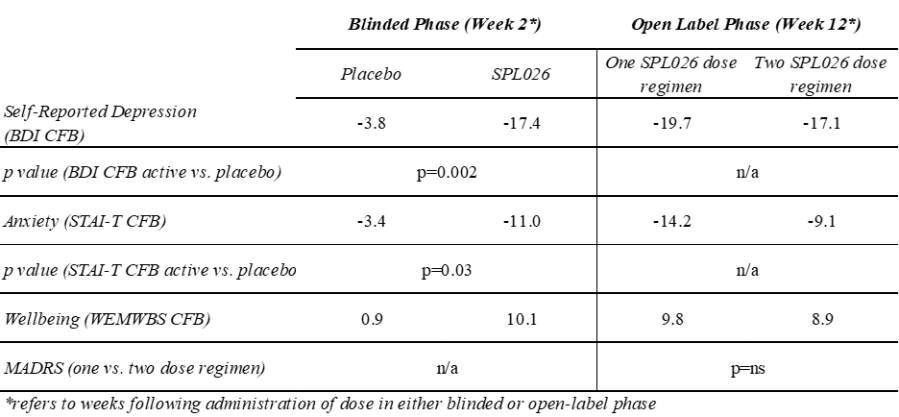
• Shareholders overwhelmingly vote in favour of Cybin’s acquisition of Small Pharma, supporting the combined company’s vision to build an international clinical-stage leader in psychedelic-based therapeutics LONDON, Oct. 19, 2023 (GLOBE NEWSWIRE) -- Small Pharma Inc. (TSXV: DMT) (OTCQB: DMTTF) (the “ Company ” or “ Small Pharma ”), a biotechnology company focused on short-duration psychedelic-assisted therapies for mental... Read More