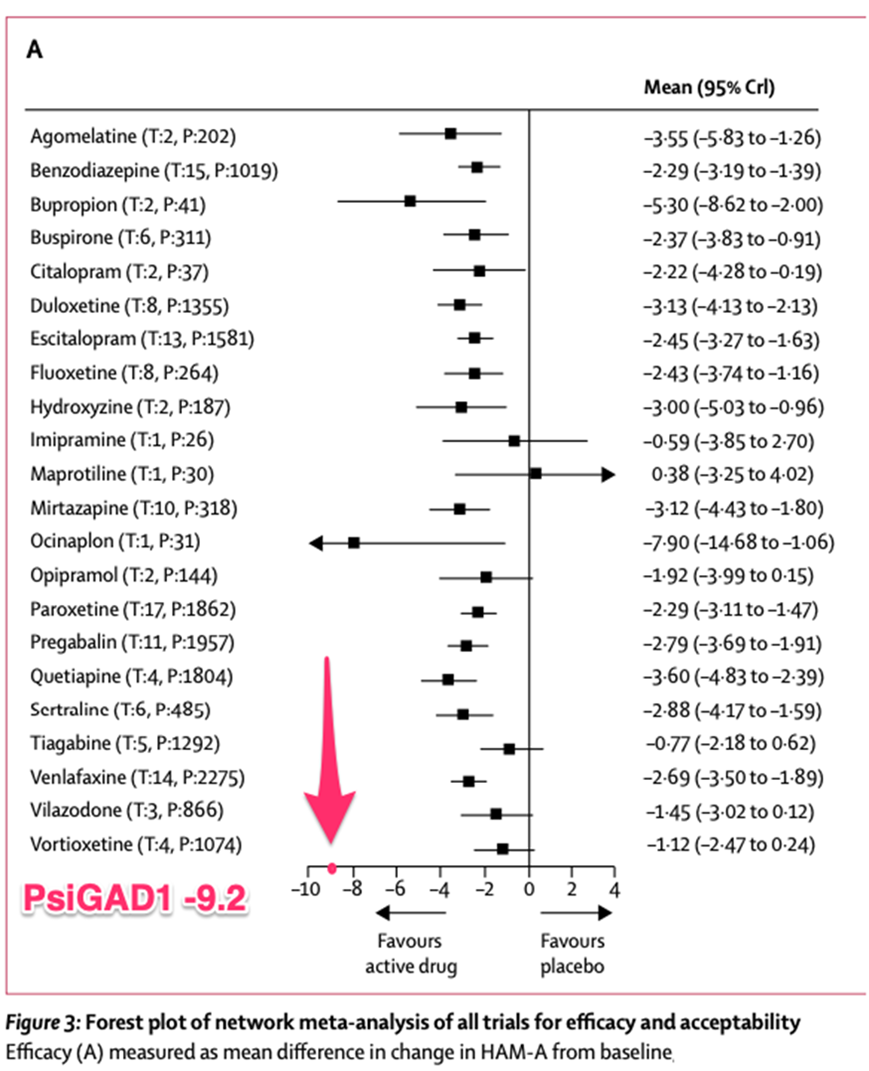
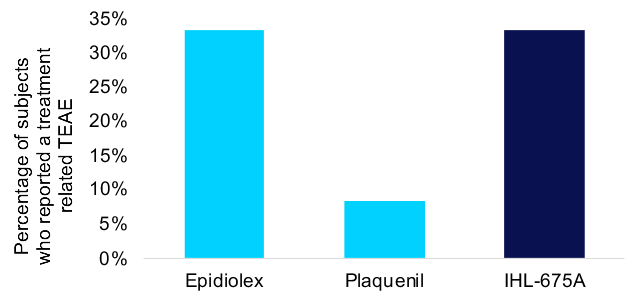
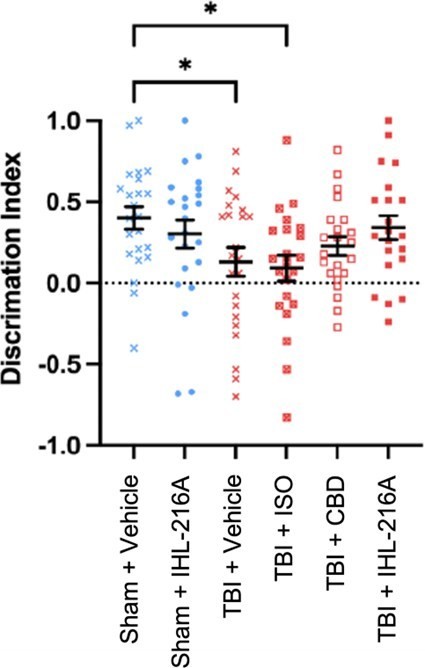
Dallas, Texas--(Newsfile Corp. - December 2, 2024) - Incannex Healthcare Inc. (NASDAQ: IXHL): Stonegate Capital Partners updates their coverage on Incannex Healthcare Inc. (NASDAQ: IXHL). During 1Q 2025 the Company reported research and development costs of $2.9M, an increase of $0.3M from 1Q24. We expect R&D costs to continue to climb as the Company focuses on getting its drug candidates across the finish line. IXHL... Read More