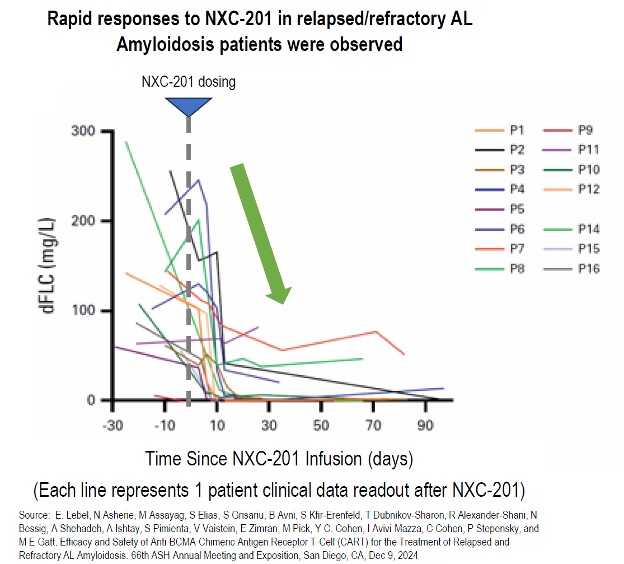
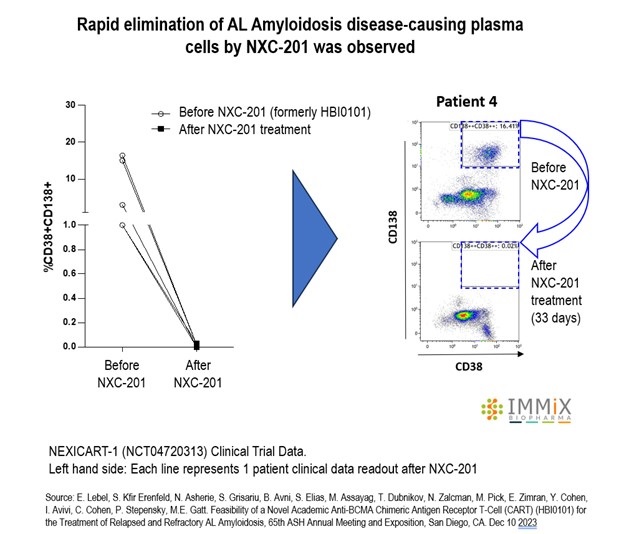
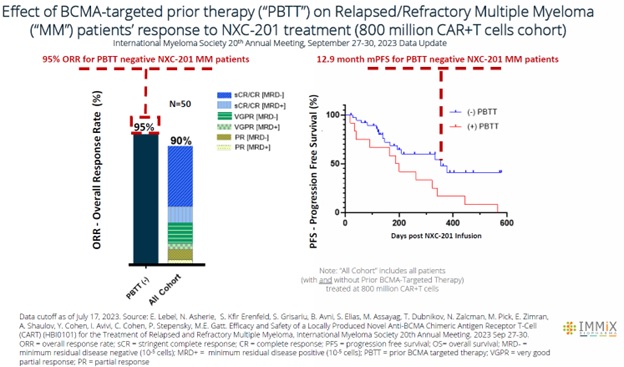
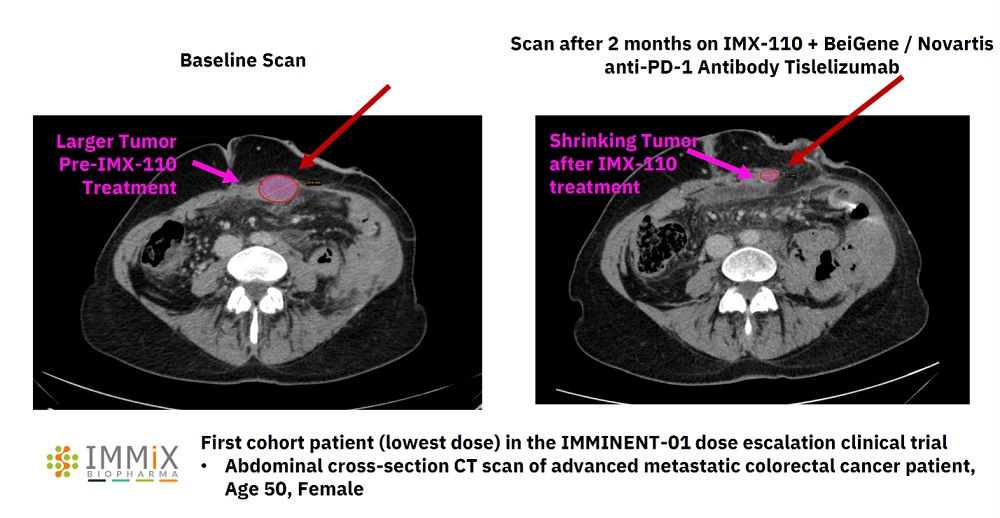
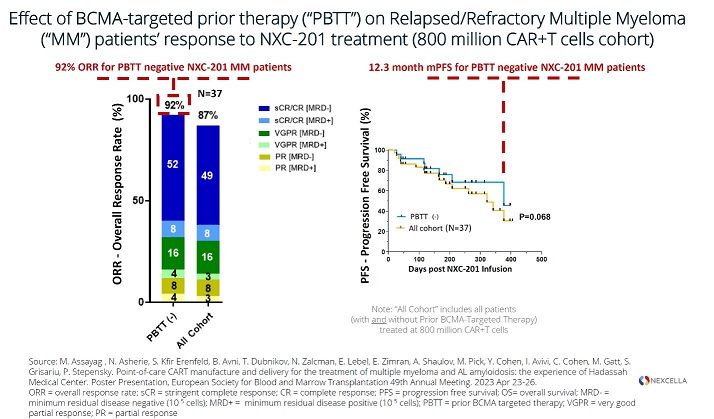
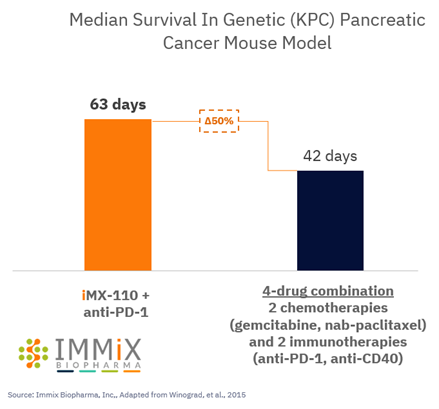
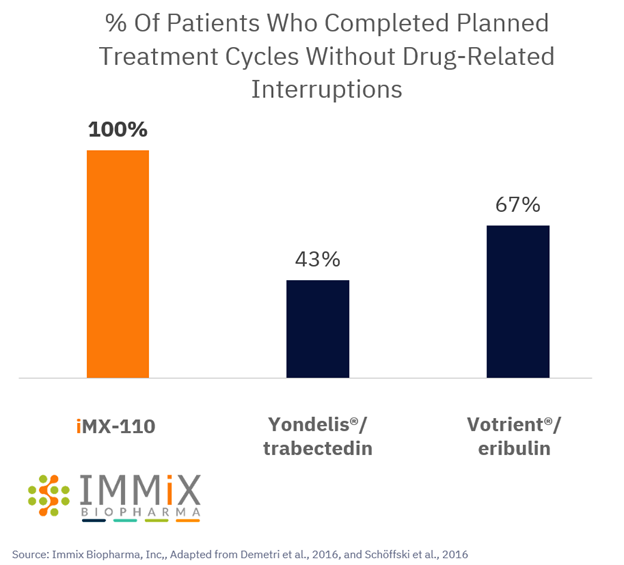
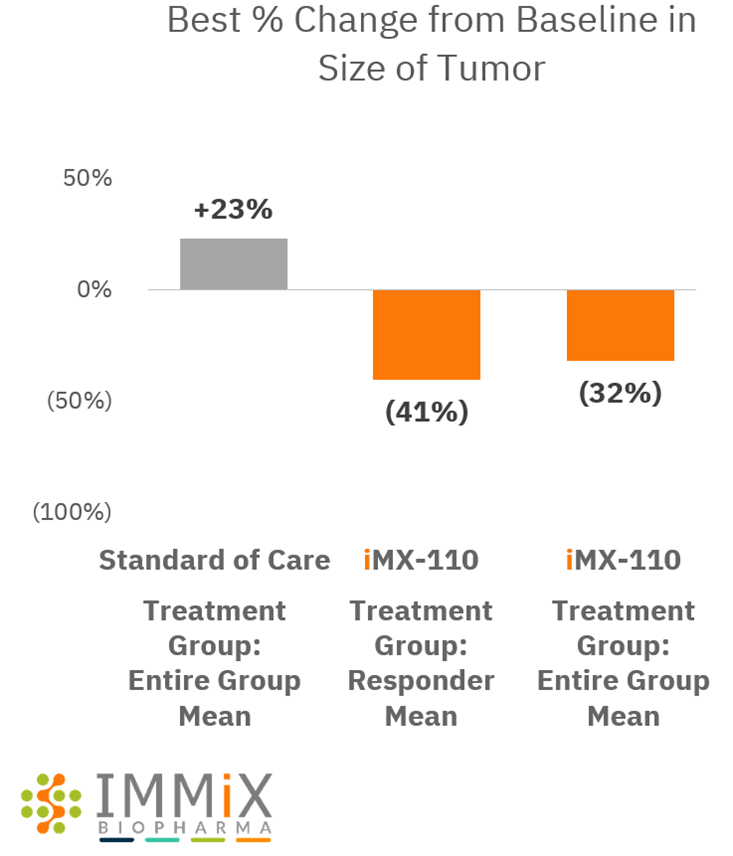
All four patients treated with NXC-201 normalized their disease markers within 30 days of dosing, of which, two are already classified as complete responders (CR), and the remaining two are bone marrow MRD negative (10 -6 ); all patients remain in response as of the data cutoff of Nov 14, 2024 Bone marrow MRD negativity... Read More