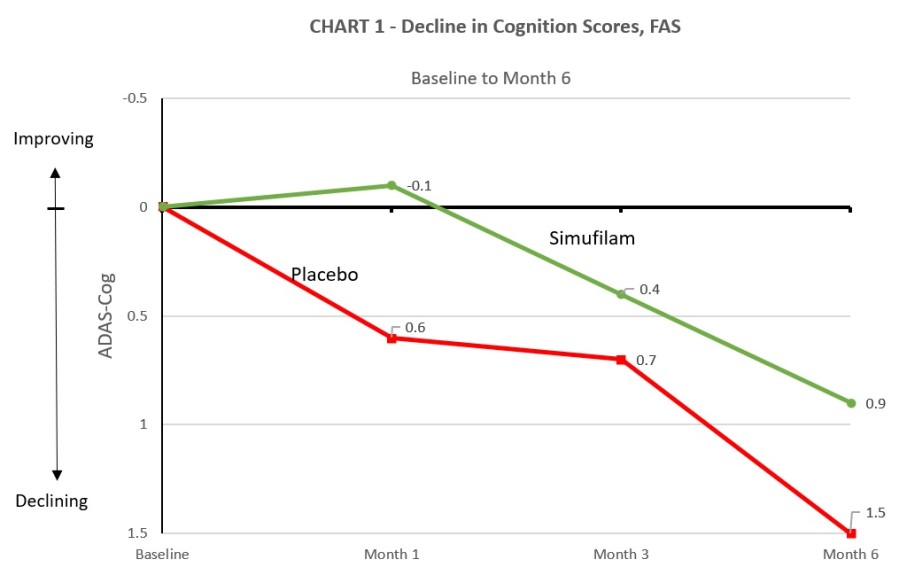
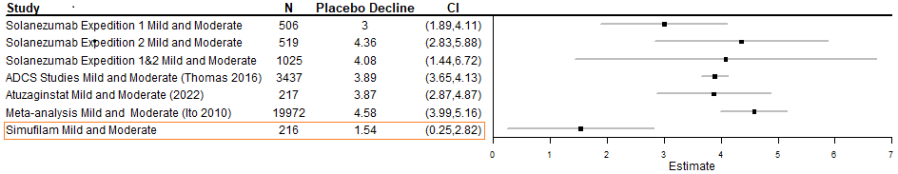
Simufilam did not show a significant reduction in cognitive or functional decline versus placebo in patients with mild-to-moderate Alzheimer’s disease in the ReThink-ALZ Phase 3 study Simufilam continued to demonstrate an overall favorable safety profile Cassava intends to present the data at an upcoming medical meeting The Company will hold a webcast today, November 25, 2024, at 8:00 AM ET AUSTIN, Texas, Nov. 25, 2024... Read More