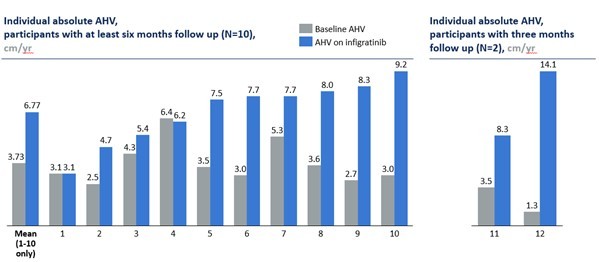
Attruby is the first and only approved product with a label specifying near-complete stabilization of TTR. Attruby has been shown to preserve the native function of TTR as a transport protein of thyroxine and vitamin A and to demonstrate benefit on cardiovascular outcomes Attruby demonstrated the most rapid benefit seen in any Phase 3 study of ATTR-CM to date: In as few as 3 months, the time to first event (all-cause... Read More