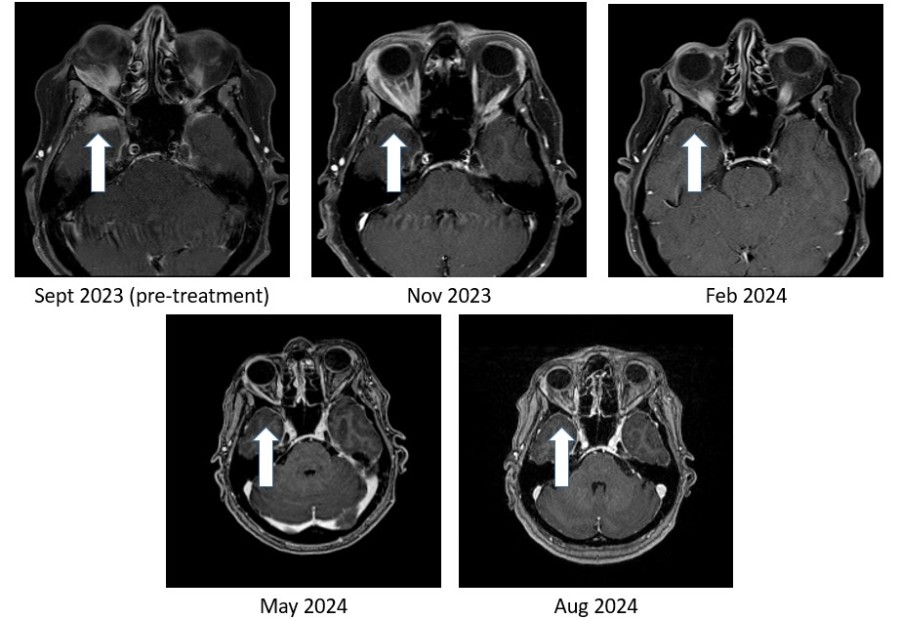
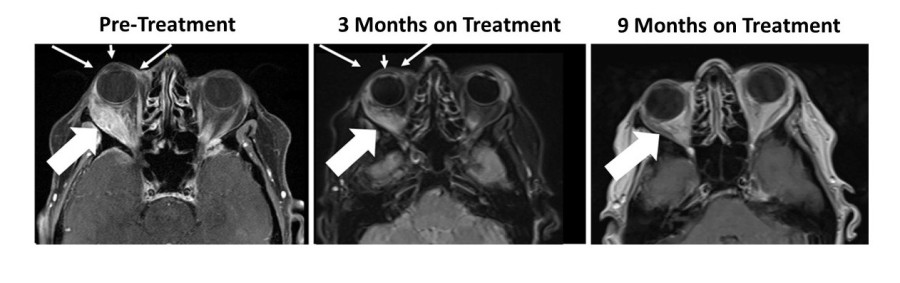
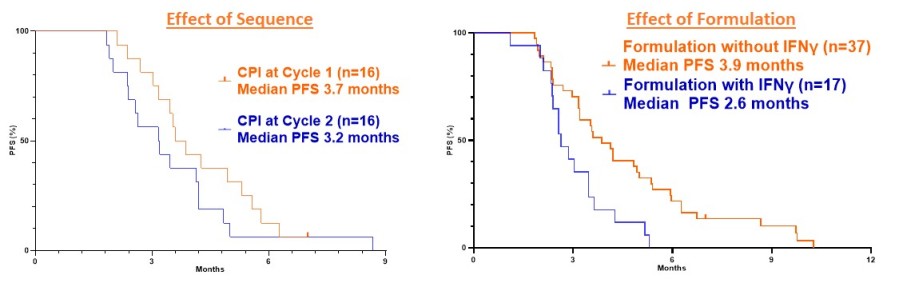
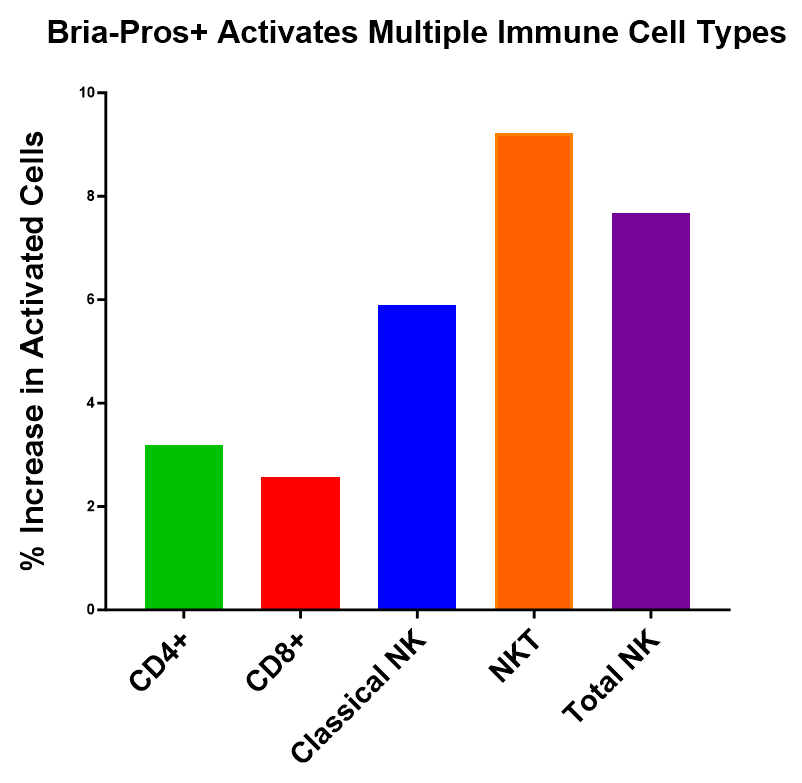
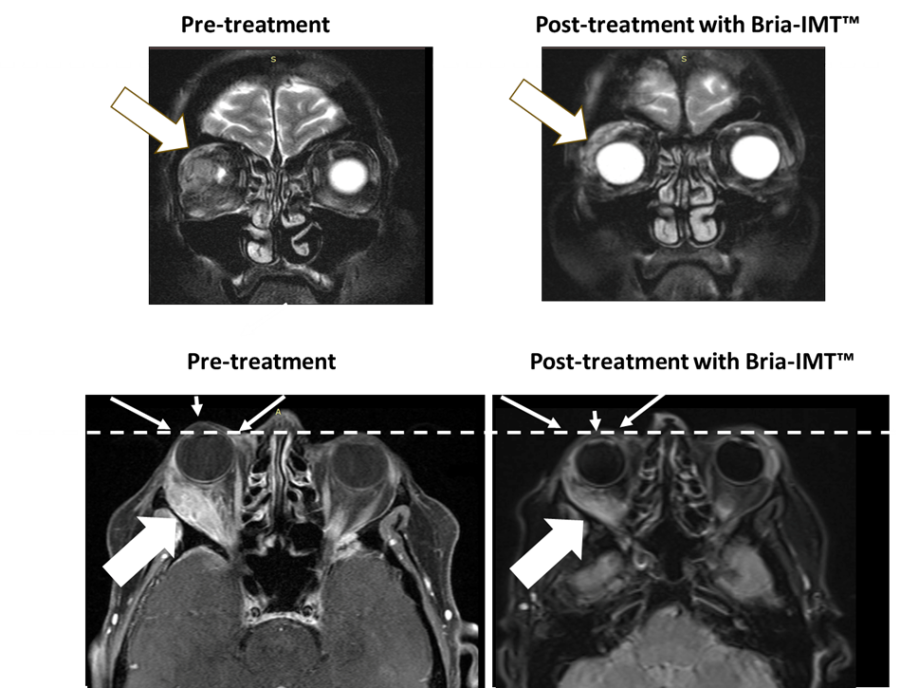
PHILADELPHIA and VANCOUVER, British Columbia, Dec. 17, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, announces that the Company will be relying on, and has satisfied all of the conditions to rely on, CSA Coordinated Blanket Order 51-931 for exemption from... Read More