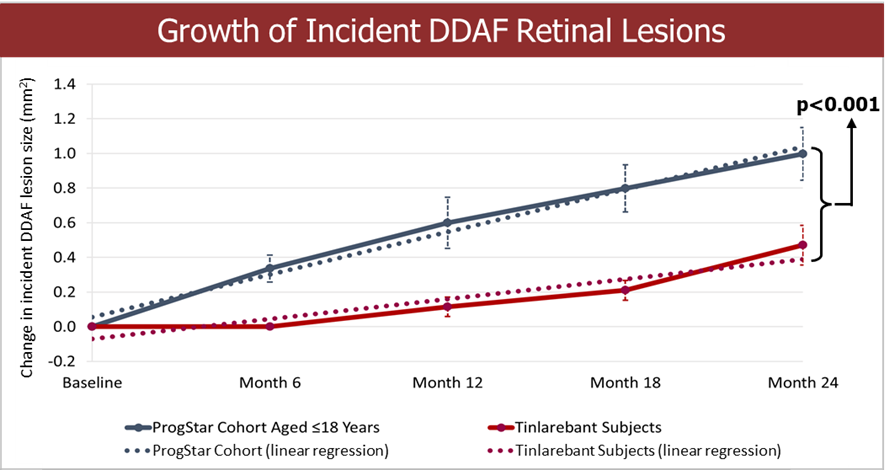
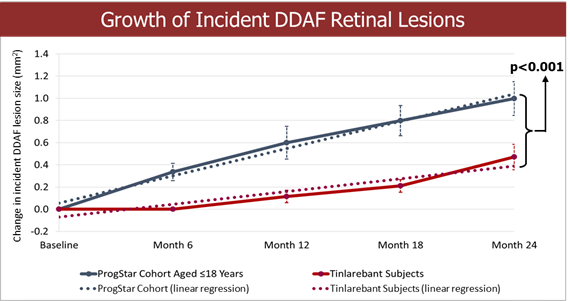
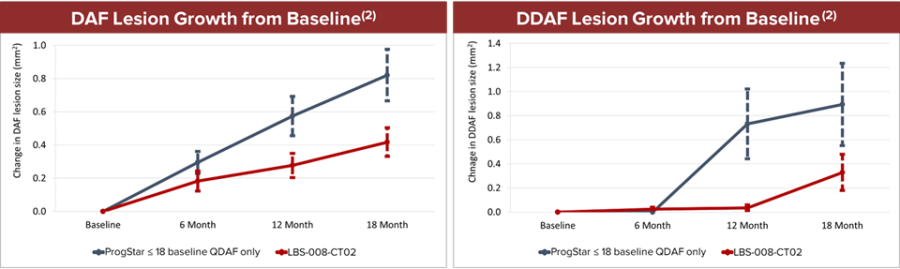
Fast Track designation indicates LBS-007’s potential in filling the unmet medical need of acute leukemia LBS-007 is the Company’s investigational medicinal product for the treatment of acute leukemia A Phase 1/2 trial in patients with relapsed or resistant acute leukemias is ongoing LBS-007, the Company’s lead asset, has been granted orphan drug designations for acute myeloid leukemia and acute lymphocytic leukemia by the... Read More